
Gap Assessment
We provide this service to organisations who already have policies, procedures, manuals,forms and work instructions, but want to determine whether or not they adhere to the appropriate standards and what steps they need to do to certify the Quality Management System.
Certification Support
We provide comprehensive support throughout the entire certification journey. Our service cover the scope of ISO9001, ISO13485 and ISO27001 standards, key activities include Quality Management System (QMS) establishments / improvements, internal audits, records maintenance, certification audits.
QMS Establishment & Implementation
We help to establish or improve quality system related documentation (manuals, policies, procedures, work instructions, forms, templates and registers) to comply with relevant standards and regulatory requirements.
Maintenance & Continuous Improvement
On-going maintance of the QMS is mandatory and it is crucial to keep all tasks up-to-date in order to maximise the effectiveness and efficiency of the QMS. Many organisations find it challenging to maintain its QMS after being certified, and it will become even more difficult facing the auditor during the annual Surveillance Audit. We offer on-going maintenance service for part or entire QMS of your organisation.
Internal Audit
Annual internal audit(s) are necessary to obtain certification and retain ISO certificates. We provide internal audit services to organisations that lack the internal resources or would like an external expertise to review and identify opportunities for improvements.
Regulatory Affairs
Provide overseas Medical Devices / IVD manufacturers with RA services support, assisting organisation to comply with the Australian Therapeutic Goods Administration (TGA) and New Zealand MedSafe requirement. Acting as your organisation’s Sponsor / Local Representative in Australia and New Zealand.
Services for Your Organisation
Instead of providing templates of our standardised services, we first listen and understand the challenges that your organisation is facing, and then we discuss with you to come up with plans to overcome those challenges or to achieve any other business goals for improvement.
Streamline QMS
Steamlining is one of the key in today's QMS, therefore all of the QMS we developed have the minimal documentation to start with. We offer full support services to organisations who wish to upgrade their QMS to the next level.
Local Regulatory Affairs Expertise
With our Regulatory Affairs Expertise in Australia and New Zealand, we have obtained regulatory approvals for products from International Medical Devices / IVD manufacturers located overseas. We also act as the Sponsor / Local Representative in Australia and New Zealand for International Medical Devices / IVD manufacturers to comply with Australian Therapeutic Goods Administration (TGA) and New Zealand MedSafe requirement.
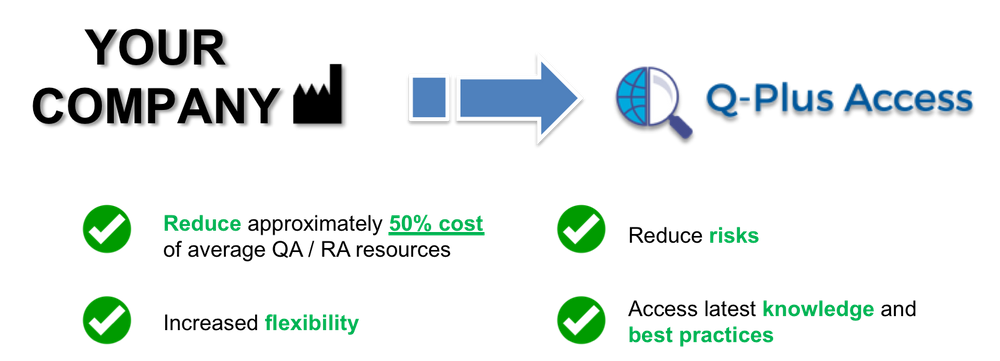
Your External Quality Assurance / Regulatory Affairs (QA / RA) Manager
Our goal is to help your company to continuously remain regulatory compliance, continuous improvement of the Quality Management System (QMS), and provide support in any other QA / RA matters that it may come up, while there is no need to worry about the high expenses for an extra full-time head count in the office. We are your External QA / RA Manager.
Henry helped us to establish and implement Quality and Information Security Management System (QISMS) that complies with ISO9001 standard and Victorian Protective Data Security Standards (VPDSS). Henry also led us to a big achievement in obtaining ISO27001 certification for Information Security field. The entire organization is operating much more effective and efficient in achieving our business goals, and with all the certifications we now hold, it gives confidence to our customers in purchasing our products and services, and helped us in winning more tenders. I highly recommend Q-Plus Access when it comes to Quality Assurance.
- Mark Kuch
CTO, Forms Express
We have been working with Q-Plus Access for a couple of years now. Q-Plus Access has acted as our Australian Sponsor, guided us through the TGA submission process and has helped us to gain our very first TGA approval that opened our door to the Australian IVD market.
- Veronica
Contact us for a private session to discuss on how we can help
© 2022 Q-Plus Access Pty Ltd. All rights reserved
